See bladder cancer in a different light
Blue Light Cystoscopy with Cysview® improves detection of non-muscle invasive bladder cancer1,2
What is Cysview?
FDA-approved Cysview makes non-muscle invasive bladder cancer (NMIBC) tumors glow bright pink under blue light during Blue Light Cystoscopy (BLC®).3
Cysview solution is placed into the bladder of a bladder cancer patient via a catheter one hour prior to BLC.3 During BLC, Cysview then allows urologists to remove cancer more completely than with standard White Light Cystoscopy (WLC) alone, because BLC with Cysview makes tumors more visible.1,2
Indication: Cysview is an optical imaging agent indicated for use in the cystoscopic detection of carcinoma of the bladder, including carcinoma in situ (CIS), among patients suspected or known to have lesion(s) on the basis of a prior cystoscopy, or in patients undergoing surveillance cystoscopy for carcinoma of the bladder. Cysview is used with the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) system to perform BLC as an adjunct to WLC.3
Who is Cysview® for?
Cysview is for use in people suspected of having, or are known to have, bladder cancer or those undergoing bladder cancer surveillance.3
It should be used during initial or repeat transurethral resection of bladder tumor (TURBT)4 and in surveillance cystoscopies.5


Blue Light Cystoscopy (BLC®) with Cysview® improves non-muscle invasive bladder cancer (NMIBC) tumor detection1,2
What evidence supports Cysview®?
Two different prospective, multicenter clinical studies looked into the effectiveness of BLC with Cysview versus standard White Light Cystoscopy (WLC) alone in detecting bladder cancer in over 300 patients with known or suspected bladder cancer.
They showed that BLC with Cysview improved tumor detection during surgery and in follow-ups:1–3
A prospective, comparative, within-patient controlled, multicenter, phase III study in the detection of Ta/T1 NMIBC in patients who had previously undergone a cystoscopy and had suspicion of or confirmed NMIBC. (n=286)
During surgery, 16% of patients had additional Ta/T1 tumors only found with BLC with Cysview.
of patients
had additional Ta/T1 tumors only found with BLC with Cysview, during surgery2,3
A prospective, comparative, within-patient controlled, multicenter, phase III study in the detection of bladder cancer during surveillance. Patients with suspected recurrence at surveillance were sent to the operating room. (n=63)
- At follow-up, only BLC with Cysview detected 21% of patients with recurrence
- During surgery, 35% of patients with CIS tumors were only found with BLC with Cysview
of patients
with recurrence were only detected with BLC with Cysview at follow-up1,3
of patients
with carcinoma in situ (CIS) tumors were only found with BLC with Cysview, during surgery1,3
Could Blue Light Cystoscopy (BLC®) with Cysview® be for me?
BLC with Cysview should be considered for patients that are:
At initial transurethral resection of bladder tumor (TURBT) on suspicion of non-muscle invasive bladder cancer (NMIBC)4
Having repeat TURBT4
Being checked to assess response to Bacillus Calmette-Guérin (BCG) therapy six weeks after completion4
Undergoing surveillance4,5
Prior to intravesical therapy when residual disease is suspected i.e., patient has not previously had BLC with Cysview5
With positive cytology and negative White Light Cystoscopy (WLC)4
Cysview may not detect all bladder tumors and is not a replacement for random biopsies.

Where is Blue Light Cystoscopy (BLC®) with Cysview® available?
Cysview is available in many locations throughout the US, with more locations being added regularly.
Use our Location Finder Tool to see where it’s available near you.
Please note, some locations may not be listed because of hospital policies that prohibit their inclusion in this type of tool.
Two types of cystoscopy
White Light Cystoscopy (WLC)
In a standard cystoscopy procedure, the light used is a regular white light – the type used to light a room. White light helps the urologist visually assess the general health of the bladder and find easily visible irregularities to be further evaluated.6
Blue Light Cystoscopy (BLC®)
When blue light technology and Cysview are available, they are used together with white light in the BLC procedure. The urologist first views the bladder with white light, then switches to blue light to see any bright pink tumor tissue areas that Cysview has caused to fluoresce.6 The urologist then switches between white and blue light to perform the necessary tumor removal.6
See the difference
Bladder images under white and blue light*
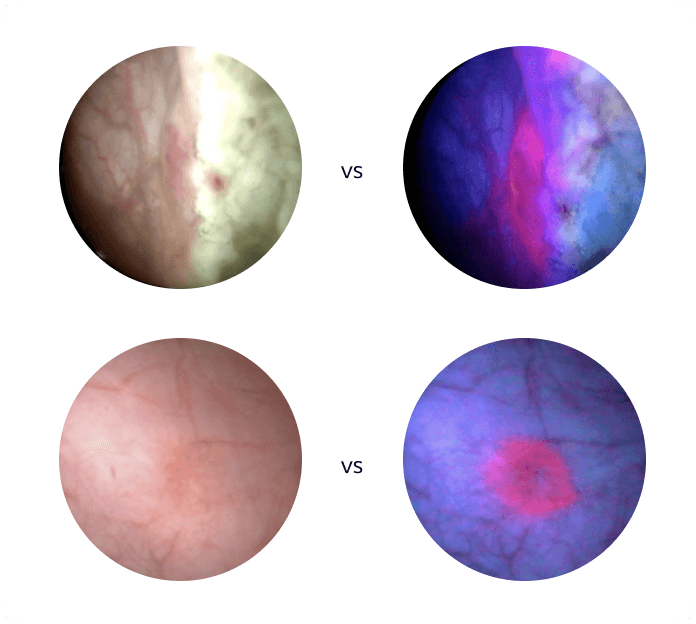
*Side-by-side images are the same area of the bladder in white and blue light.
Cysview may not detect all malignant lesions.
Safety information
Any procedure may have some risks. You should consult your urologist regarding the risks and benefits of this procedure.
The most common adverse reactions reported in patients who received Cysview were:3
- Bladder spasm
- Dysuria
- Hematuria
- Bladder pain
The following adverse reactions have been voluntarily reported during post-approval use of Cysview. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure:3
- Anaphylactoid shock
- Hypersensitivity reactions
- Bladder pain
- Cystitis
- Abnormal urinalysis

References
1. Daneshmand S, Patel S, Lotan Y, et al. Efficacy and Safety of Blue Light Flexible Cystoscopy with Hexaminolevulinate in the Surveillance of Bladder Cancer: A Phase III, Comparative, Multicenter Study. J Urol. 2018;199(5):1158–1165. 2. Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate Guided Fluorescence Cystoscopy Reduces Recurrence in Patients with Nonmuscle Invasive Bladder Cancer. J Urol. 2010;184(5):1907–1913. 3. Cysview [prescribing information]. 2019:1–4. 4. Daneshmand S, Schuckman AK, Bochner BH, et al. Hexaminolevulinate Blue-Light Cystoscopy in Non-Muscle-Invasive Bladder Cancer: Review of the Clinical Evidence and Consensus Statement on Appropriate Use in the USA. Nat Rev Urol. 2014;11(10):589–596. 5. Lotan Y, Bivalacqua TJ, Downs T, et al. Blue Light Flexible Cystoscopy with Hexaminolevulinate in Non-Muscle-Invasive Bladder Cancer: Review of the Clinical Evidence and Consensus Statement on Optimal Use in the USA - Update 2018. Nat Rev Urol. 2019;16(6):377–386. 6. Urology Group of Florida. What is Blue Light Cystoscopy? Available at: https://www.urologygroupfl.com/post/what-is-blue-light-cystoscopy. Accessed March 2024.