See The Difference Cysview Makes
Bladder images under white and blue light
Standard White Light Cystoscopy

Blue Light Cystoscopy
with Cysview
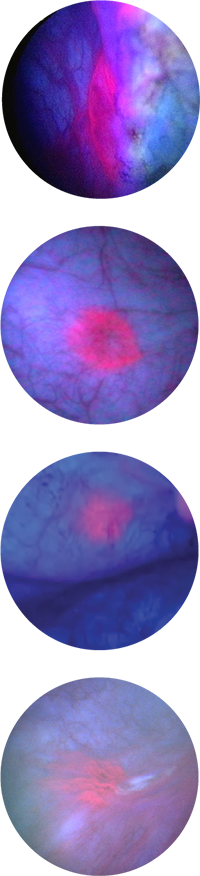
Cysview may not detect all malignant lesions. False-positive fluorescence may occur due to inflammation, cystoscopic trauma, scar tissue, previous bladder biopsy, recent BCG immunotherapy or intravesical chemotherapy.
Cysview is not a replacement for random biopsies or other procedures used in the detection of bladder cancer.
Please see Full Prescribing Information.
For more information review the Important Risk & Safety Information below.


