Required Equipment
Partnering with KARL STORZ Endoscopy-America Inc.
Cysview is FDA-approved for use with the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) system.1
Blue Light Powered by SAPHIRA™
Launched in September 2022, the Blue Light Powered by SAPHIRA™ PDD system offers state-of-the-art image quality and practical features to make the technology more user friendly.
Equipment features and benefits
- High-definition image enables razor-sharp image quality for greater precision in the blue-light mode.
- LED light source provides more consistent light quality that won’t degrade over time
- Fiber-optic cable gives you convenient autoclaving options
- CHROMA setting allows more visualization of vascularity
- Ergonomic camera head offers blue-light intensity control
Procedure benefits
- Simplified set-up process — There’s no more start-up sequence.
- More reliable parts — More durable light source and cable, which means fewer surprises during a case.
- Even better visibility — When you can see better, you can have more confidence in the quality of the TURBT.
To perform Blue Light Cystoscopy (BLC®) with Cysview® as an adjunct to White Light Cystoscopy, you will need the KARL STORZ PDD system components listed below.
For more details, download the equipment overview.
Drug and Device Support Resources
Urologists who perform Blue Light Cystoscopy with Cysview receive training on both the imaging solution and the technology. Staff involved in the process also receive appropriate training.
While there is a learning curve associated with gaining expertise in this technology, there are help and resources always available.
The Operating Room Guide provides important information on the set-up and use of the non-SAPHIRA™ equipment, including troubleshooting steps.
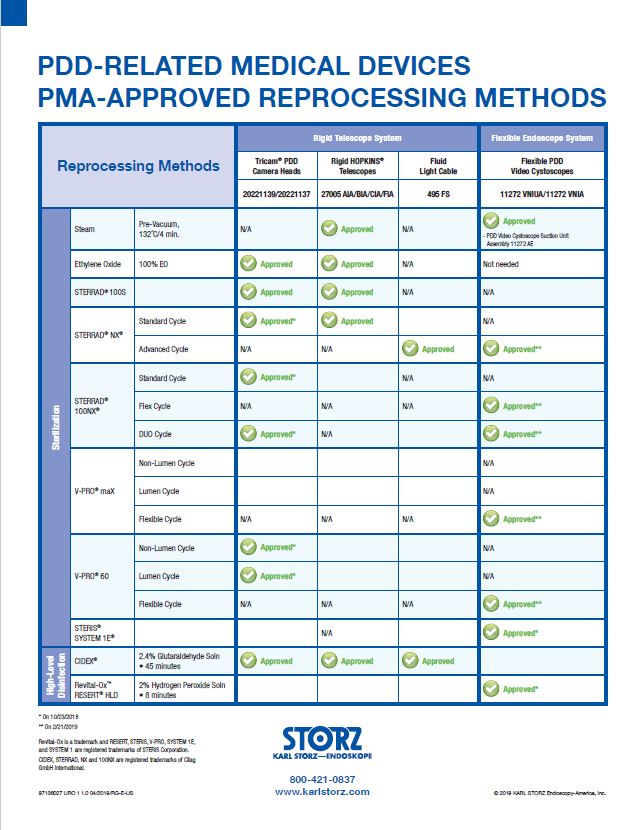
The PDD Reprocessing Guide is a quick and easy reference tool for understanding which reprocessing methods are PMA-approved for which PDD-related medical devices.
1 Cysview [prescribing Information]. 2019:1-4.