Communication Toolkit
This Toolkit is designed to help you and your institution raise awareness about bladder cancer and the availability of Blue Light Cystoscopy with Cysview® at your location.
Cysview is the only FDA-approved agent for use in Blue Light Cystoscopy. it is an optical imaging agent indicated for the detection of non-muscle invasive bladder cancer, including carcinoma in citu (CIS), in patients:
- suspected or known to have lesion(s) based on a prior cystoscopy or
- undergoing surveillance cystoscopy for carcinoma of the bladder.
Provided in this Toolkit are EDUCATIONAL RESOURCES to help with:
- Educating Local Healthcare Professionals
- Building Community Awareness
- Educating Patients
- Media Relations
- Website Content and SEO Keywords
- Social Media
Introduction to this Educational Resource
This Communication Toolkit is designed to help you publicize the availability of Blue Light Cystoscopy with Cysview to a variety of audiences who will find this news relevant – and even life-changing.
While the information in this Toolkit can be used by anyone, it is organized by different user types to create greater relevance for each user.
Feel free to explore all the resources available or simply jump to the section that best matches your situation. Information for:
- Marketing Personnel introducing the availability of BLC with Cysview
- Marketing Personnel promoting the availability of BLC with Cysview
- Individual urologists who want to promote their use of BLC with Cysview
- Anyone who would like help and support from the Cysview Marketing Team
Available templates are written from two different perspectives, when appropriate:
- Introducing the availability of BLC with Cysview
- Promoting the availability of BLC with Cysview
You are invited to copy and paste the templates provided in this Toolkit and make them your own on facility stationery; or you can simply use these suggestions as a guide to create your own unique marketing program. We hope you find this Toolkit useful in some way.
Educating Local Healthcare Professionals
Introduction Letter

Building Community Awareness
Event Email
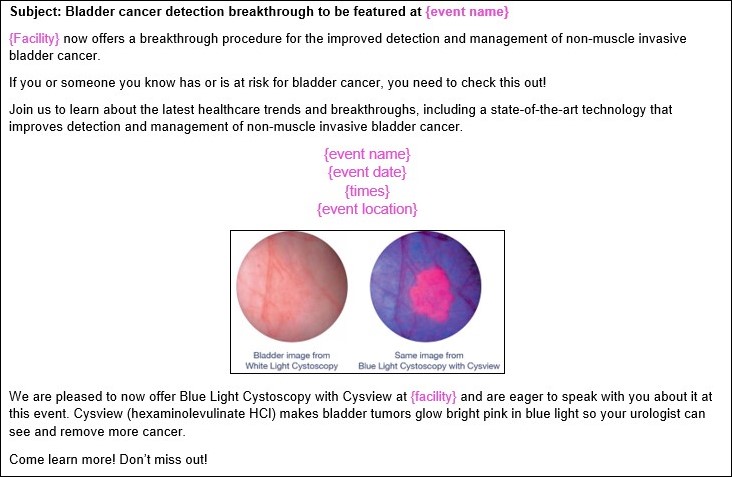
Media Relations
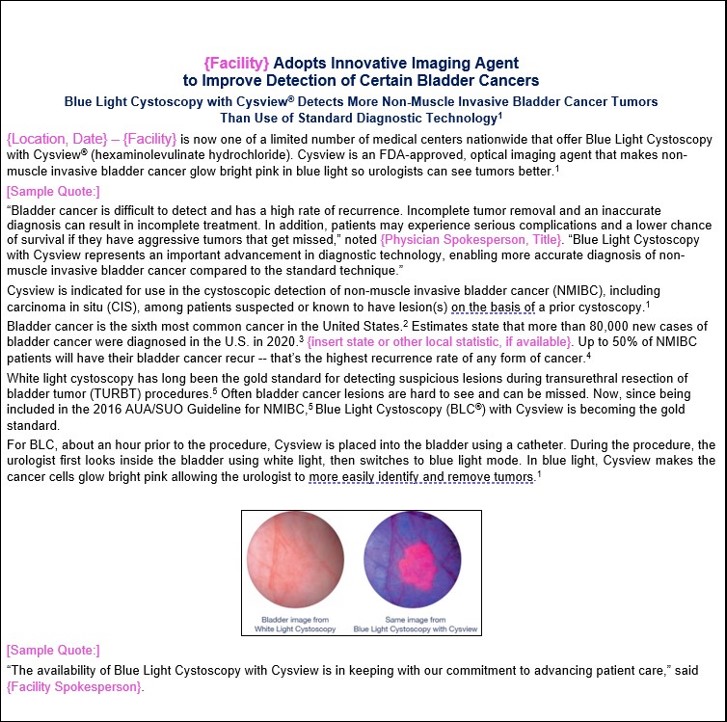
Press Release
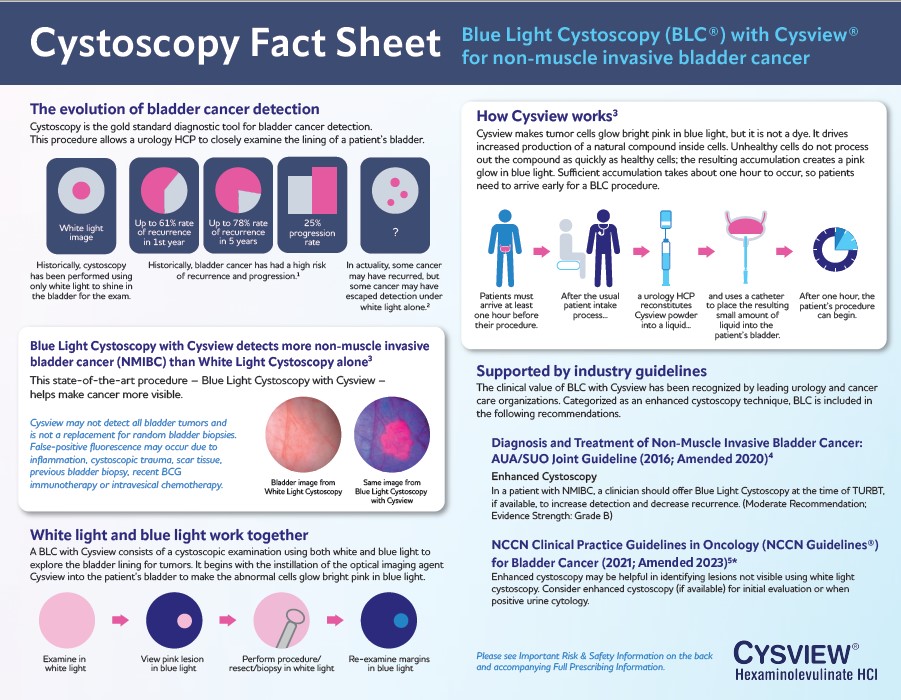
Fact Sheet
Educating Patients
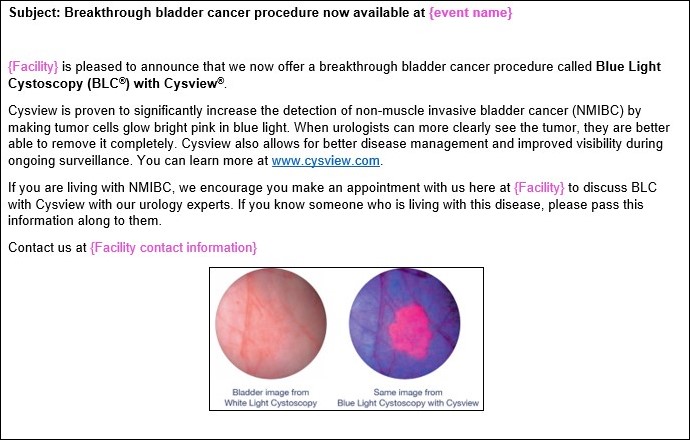
Email to Patients
Copy for Patient Newsletters
FAQ

Patient Slide Presentation

Patient Brochure

“Cysview Available Here" Poster & Tabletop Sign

Other Assets
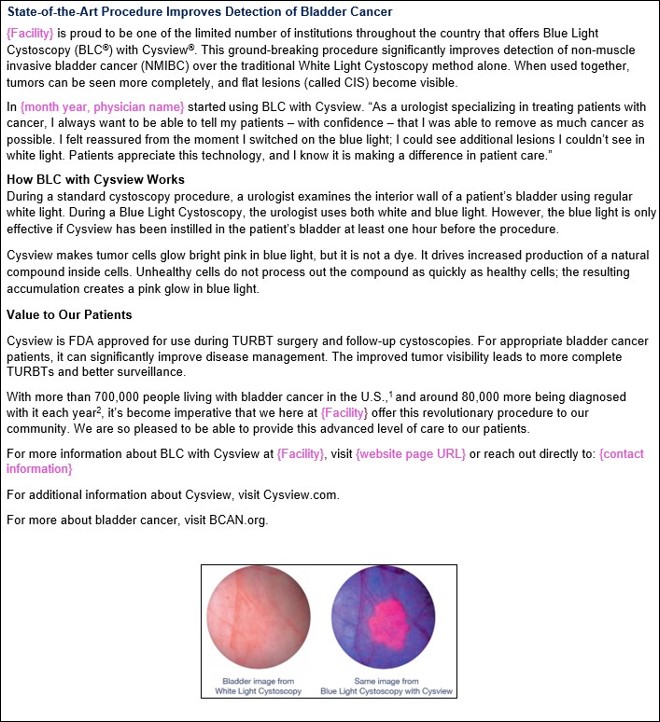
Clinical Images
Brand Images

